If you are interested in our Photon Counting Toolkit, please go to pctk.jhu.edu.
We wish to develop technologies that enable quantum leaps in medical imaging and change the game. We identify clinically important problems and tackle them genuinely by taking theoretically sound engineering approaches, respecting physics and math, and taking into account physiology and medicine. It has not been an easy work, but we truly believe that it is an important approach for making real-world impact.
Our research focuses on X-ray CT and X-ray systems, as we love x-ray-based imaging has a good mixture of physics, math, and engineering aspects. We have been pioneering the two research areas–photon counting CT and “IPEN.” We are honored to be recognized as one of the leading research labs for the fields.
Spectral, photon counting detector (PCD) CT
PCD model-based algorithms
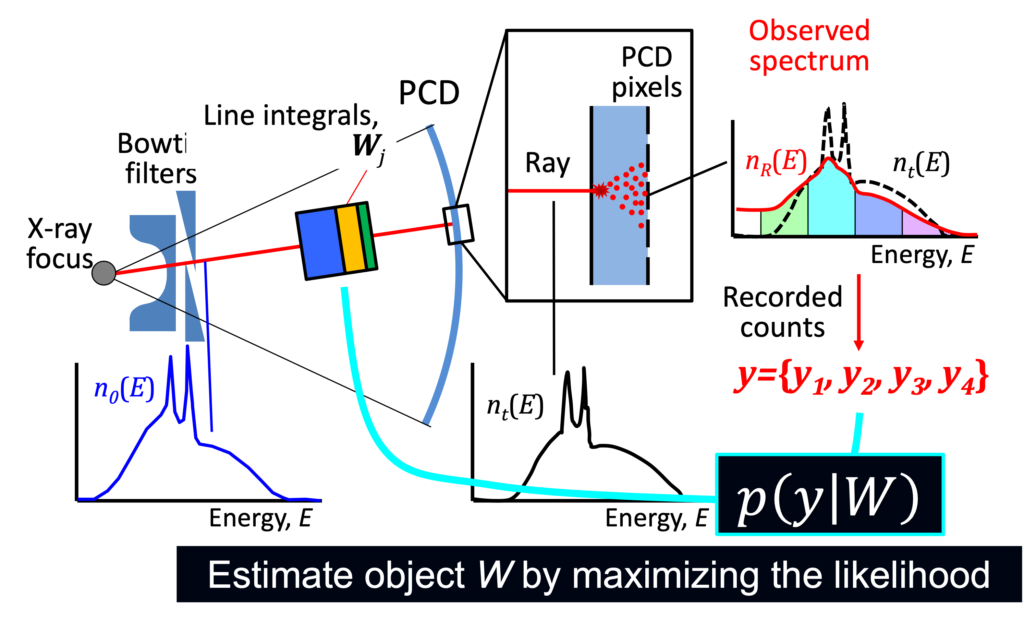
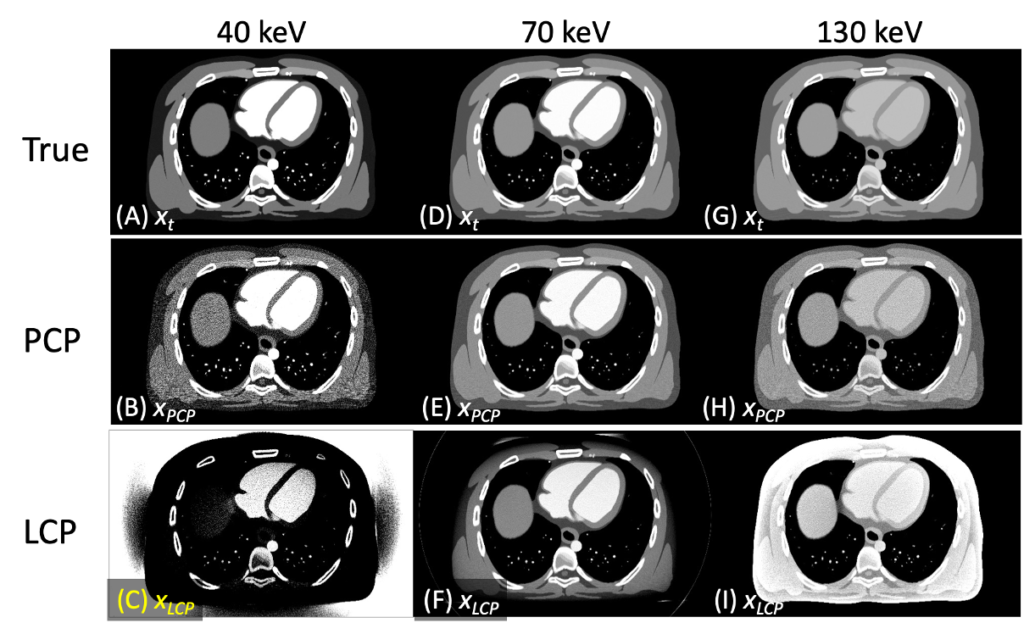
We are developing algorithms for quantitative spectral CT imaging, and integrating good PCD physics models is critical for the success. As outlined in modeling work, PCDs are not flawless, and if left uncorrected, distorted spectra would result in biases and artifacts. Our PCP algorithms integrate a PCD model into the forward imaging chain and compensate for the effect during the image reconstruction process (top figure). This approach puts the complex PCD flaws into a well-posed problem framework such that we can offset the effect and address biases (bottom figure) [see this paper, this, and this].
A new class of spectral imaging is joint estimation of tissue types and attenuation maps [see this paper]. Instead of performing multiple steps in image recon, image segmentation, and tissue type analysis, we propose to perform them jointly, and hence, to improve the accuracy of each step. We believe that spectral PCD CT provides us so many opportunities to explore, and we started scratching the surface.
Grant support: Siemens, Canon
Physics and detector modeling
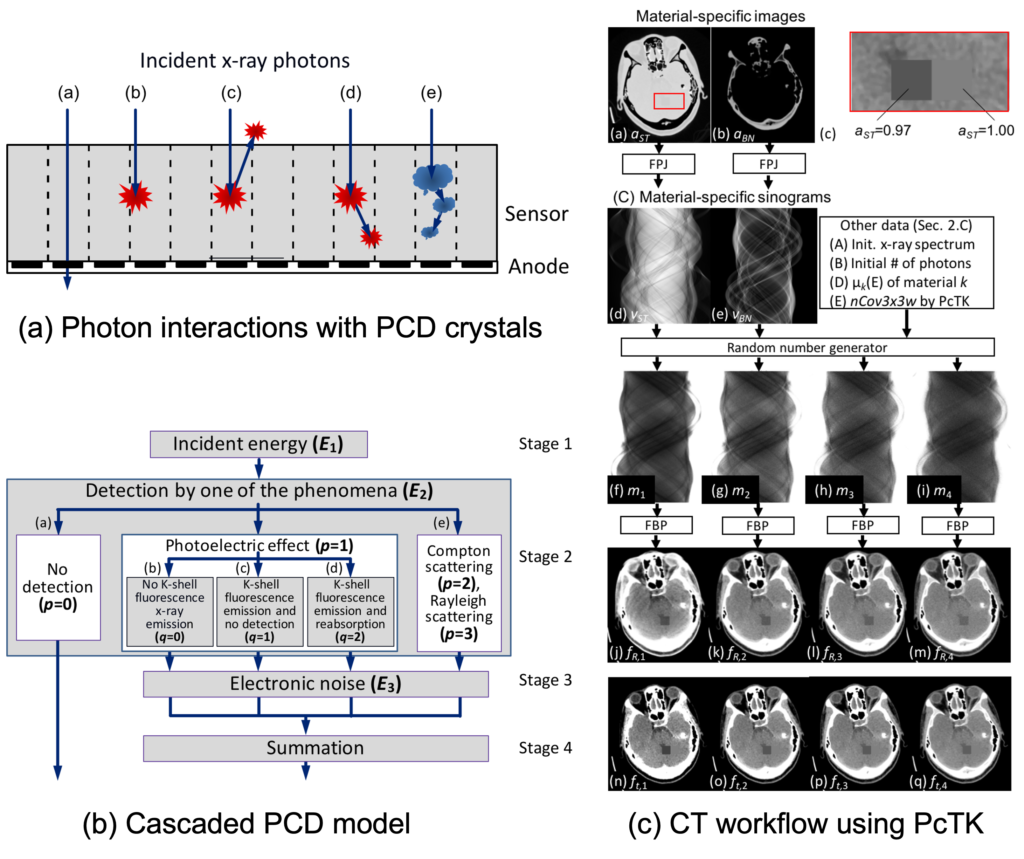
We are proud to be the front runner of this effort. PCDs are not flawless due to imperfect crystals, detection physics, limited detector speed, and detection schemes implemented. It is critical to have a good detector model for studying PCD data, developing algorithms that work well with actual PCDs, evaluating performances, designing detectors, etc. The model has to be simple enough to be tractable but also accurate enough to be practically useful. Almost 20 years ago, at the beginning of our PCD CT work, we decided to take on this challenge for the algorithm development, and we have successfully modeled most of critical phenomena and mechanisms such as charge sharing, pulse pileup, energy–voltage response, and electronic noise. We collaborated with Siemens and made our Photon Counting Toolkit program (PcTK, pctk.jhu.edu) available to academic researchers in 2018 and have released more than 118 licenses in 17 countries. See this paper, this, and this.
Grant support: Siemens, Canon
Spectral coincidence counting
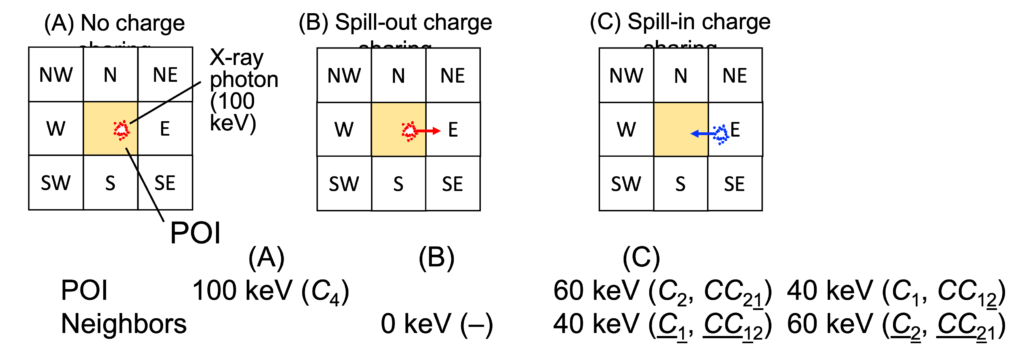
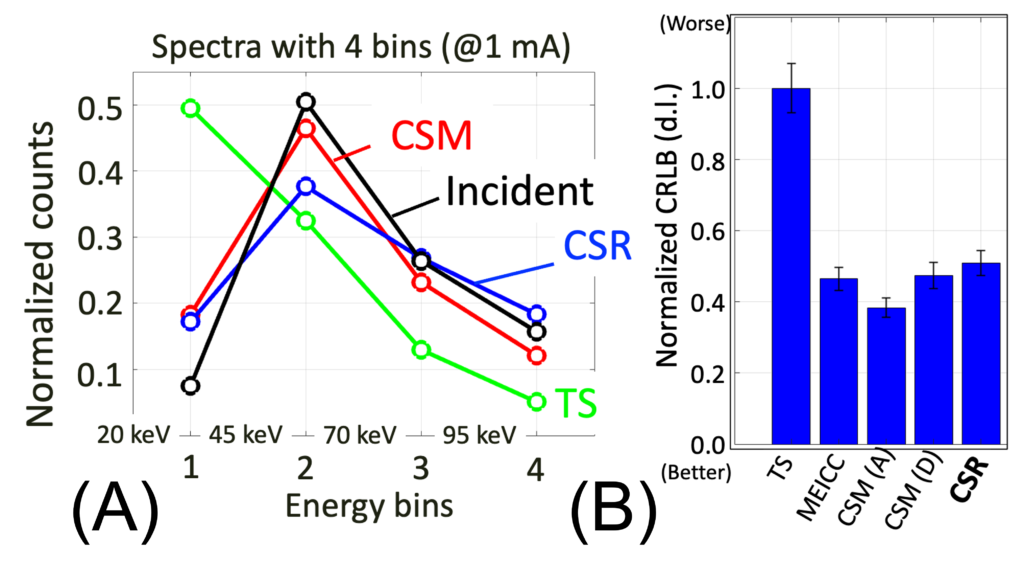
Hardware is not our strengths, but when we get an intriguing idea, we should work on it. While listening to Scott S. Hsieh’s presentation at a workshop and discussing it with him after the session, an idea of a new photon detection scheme occurred to Ken, which was later named MEICC or spectral coincidence counter.
When an x-ray photon hits a detector between two pixels, the energy is shared between them (top figure, B and C). It is called charge sharing. By recording the coincident events with energies with MEICC, one can undo the charge sharing even after data are acquired and read-out (bottom figure). We believe this is a critical technology for the next generation of PCDs and have been studying since. See this paper, this, this, and this conference presentation, and this.
Grant support: NIH R21 EB029739, NIH U01 EB037558
Scintillator-based PCD
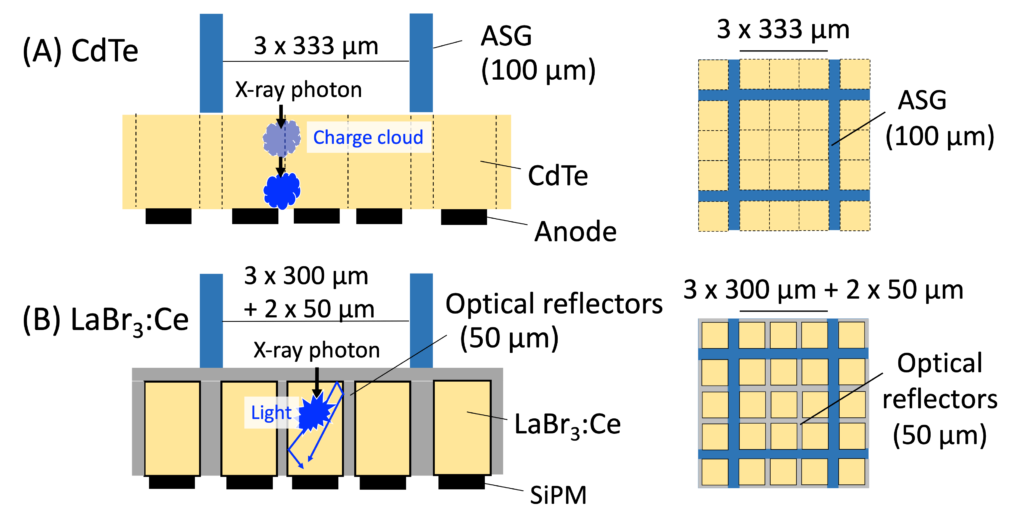
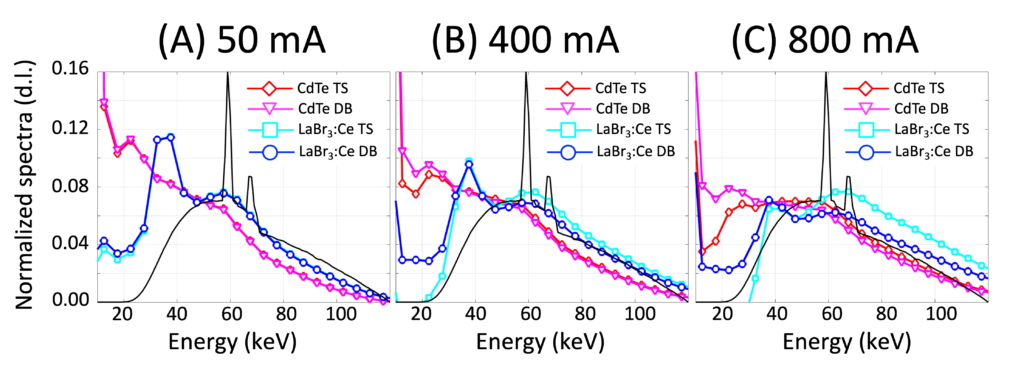
We were intrigued and excited when we learned that a few research groups were investigating the use of old CT technologies, scintillators, for photon counting detectors. Scintillators have been used for energy integrating detectors (EIDs) and previously thought to be too slow for PCD CT. Well, not any more! Recent studies have demonstrated that some high-quality scintillators such as lanthanum bromide doped with cerium (LaBr3:Ce) have comparable speed to CdTe. Moreover, our preliminary investigation revealed that scintillator-based (Sci–)PCD CT has critical advantages over CdTe PCD CT. We are excited about the potential and this thinking out-of-box approach. See this paper.
Grant support: Pending
Perfusion CT, without CT (We use an x-ray angio system)
IPEN algorithms
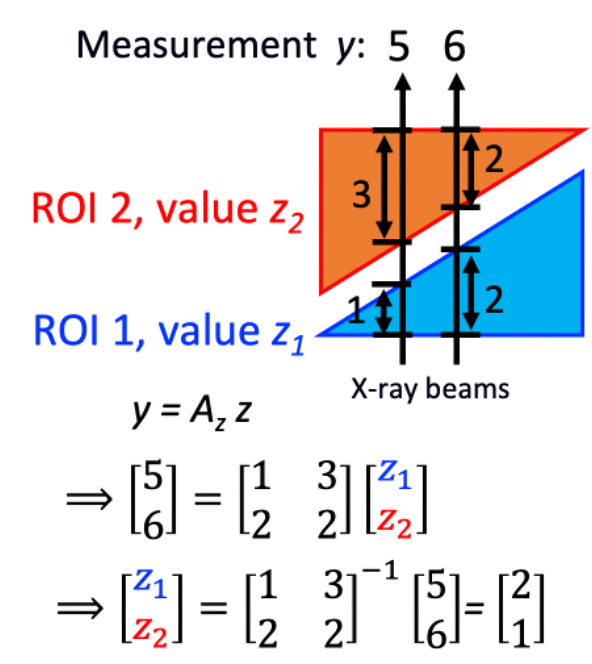
We are changing the world. We are developing IPEN algorithms; IPEN stands for Intra-operative PErfusion imaging using angiography system with No gantry/C-arm movement) and it estimates 3-D perfusion. We would think that it is impossible to do cross-section image reconstruction of the object from one projection obtained at one angle. But, what if the object can be divided into multiple regions-of-interest (ROIs) and the ROI shapes are known? What if each ROI has a uniform value?
That is the core of IPEN algorithm. Two x-ray beams are sufficient to estimate values for 2 ROIs, right? If the object has 100 ROIs, we only need 100 x-ray beams. An x-ray detector has about a million pixels (=1,000×1,000), a lot more than 100. One projection (with 1 million x-ray beams) is sufficient to estimate 100 ROI values. Now it is a well-posed, well-conditioned problem. We are developing the IPEN algorithm for general or specific cases, when a patient moves, when only digital subtraction angiography data are available, etc. See this paper and this conference presentation.
Grant support: NIH R21 EB029049, NIH R01 NS126256, JHU Discovery Award 2025
Brain perfusion for ischemic stroke
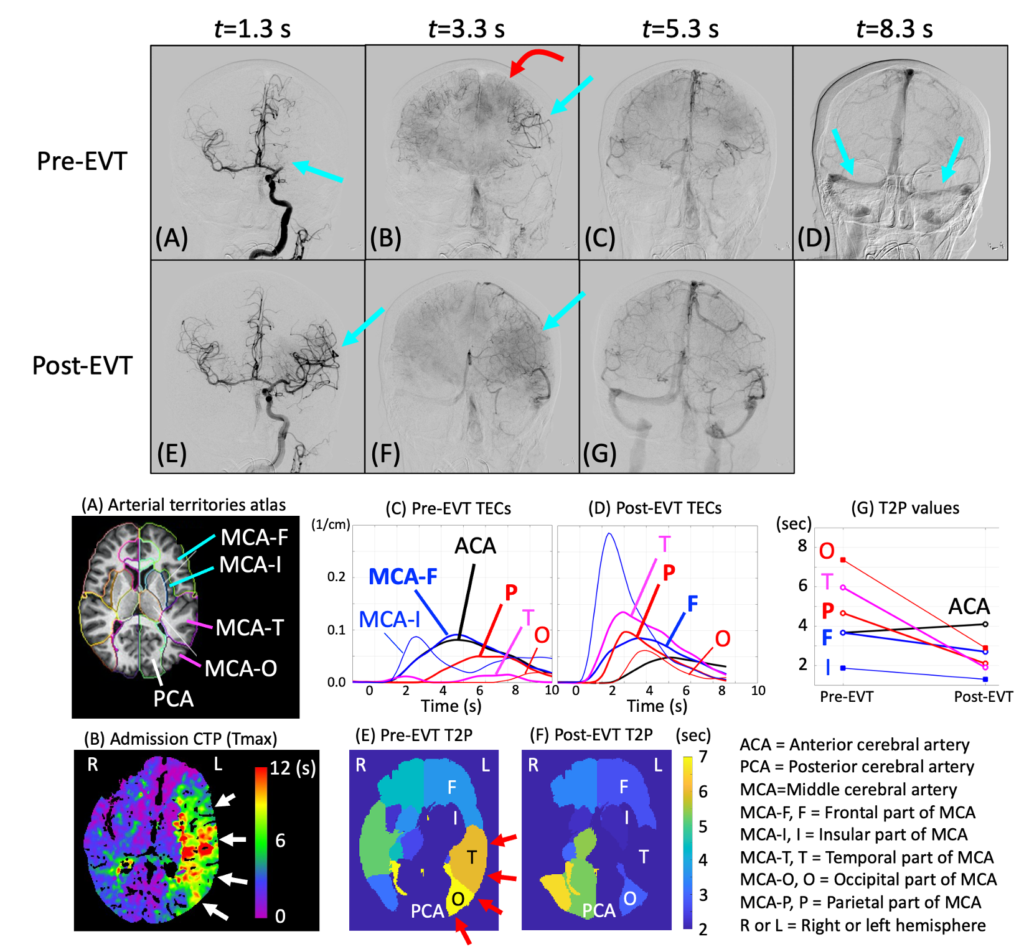
We are developing a method that uses the IPEN principle and assess brain perfusion for stroke patients. A pilot study showed very encouraging results. When fully developed, this method will consist of 5 steps and the IPEN is only 1 of the six. We have developed the IPEN stroke version 1 and computer simulation showed excellent results. See this exciting conference presentation. We are working on version 2 development for clinical data.
Grant support: NIH R21 EB029049, NIH R01 NS126256, JHU Discovery Award 2025
Liver perfusion for Trans-arterial chemo-embolization (TACE)
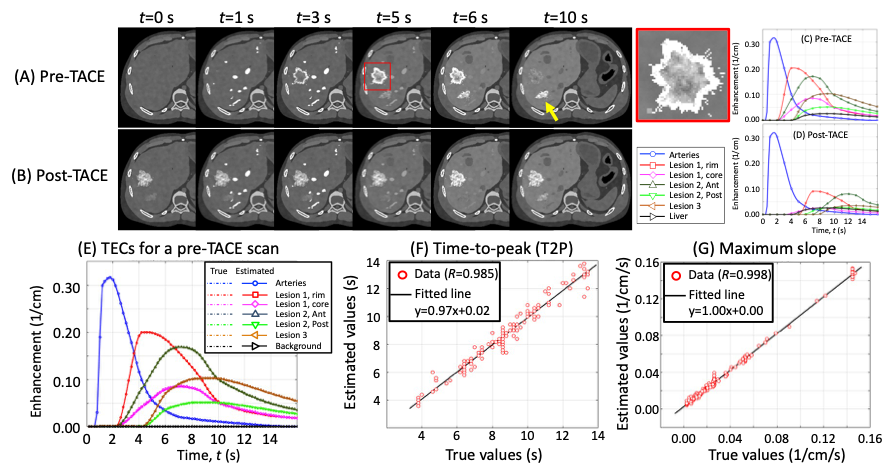
Wnterventional liver cancer treatment attacks tumors by blocking the arterial blood supply and either infusing anti-cancer drug or radioactive particles. Assessment of the blood perfusion to the targeted and surrounding areas will be invaluable to maximize the treatment efficacy. Computer simulation studies show that IPEN will allow for accurate perfusion assessment [see this paper]. See this exciting conference presentation. We plan on further development with clinical patient data.
Grant support: NIH R21 EB029049

